How To Work Out Relative Formula Mass
In absolute units protons and neutrons have masses on the order of 10 27 kilograms which is a billionth of a billionth of a billionth of a kilogram and electrons have even smaller mass of about 10 30 kilograms about a thousand times less than a. Atoms have very little mass.
how to work out relative formula mass is important information accompanied by photo and HD pictures sourced from all websites in the world. Download this image for free in High-Definition resolution the choice "download button" below. If you do not find the exact resolution you are looking for, then go for a native or higher resolution.
 Solved Question 5 Calculate The Relative Formula Mass For
Solved Question 5 Calculate The Relative Formula Mass For
Work out how many atoms of each element.

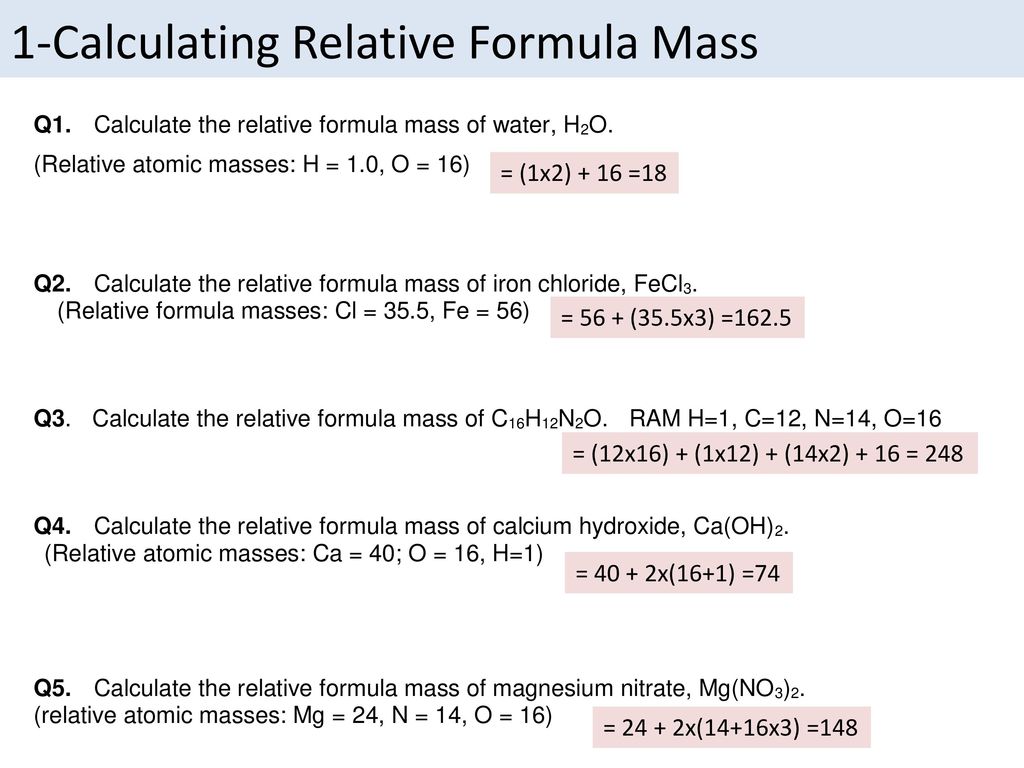
How to work out relative formula mass. The relative formula mass of a compound is calculated by adding together the relative atomic mass values for all the atoms in its formula. The method on the left takes each small group in the molecule and works out its mass first. The numbers of particles in an atom can be calculated from its atomic number and mass number.
Baca Juga
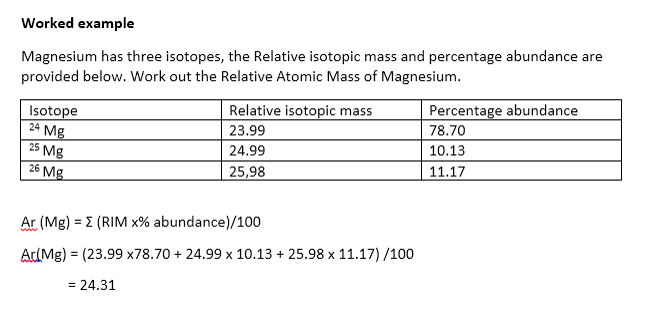
This simply means the calculation is performed using relative atomic weight values for the elements which are based on the natural isotopic ratio of elements found in earths atmosphere and crust. The a r of an element. So instead of using their actual masses in kilograms their relative atomic masses are used.
Is the mean mass of its atoms compared to 112 th the mass of a carbon 12 atom. Which method you use is up to you as you get the same answer either way. It exists to simplify the process of working out the mass of an atom or molecule.
Moles are units used to measure substance amount. The relative atomic mass of an element symbol a r is the mean mass of. The relative formula mass of a substance made up of molecules is the sum of the relative atomic masses of the atoms in the numbers shown in the formula.
Calculate the relative molecular mass of ammonium sulphate. Chemistry how to calculate relative molecular mass relative formula mass percentage mass percent mass of an element in a compound percent mass of water in a compound examples and step by step solutions. Relative mass is an important concept in chemistry.
A related term you should know is relative formula mass relative formula weight. The method on the right counts up all the atoms of each type separately. Atoms consist of a nucleus containing protons and neutrons surrounded by electrons in shells.
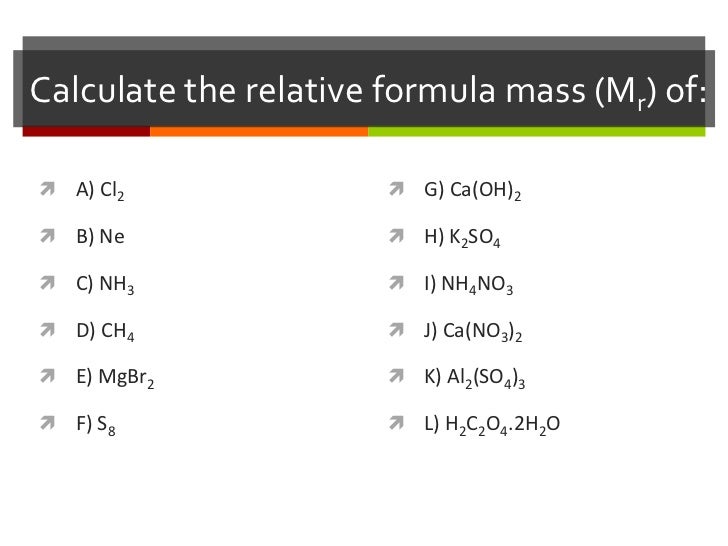
Calculating relative formula mass relative formula mass is given the symbol m r.
 The Masses Of Chemicals Learning Together Ks4 Science
The Masses Of Chemicals Learning Together Ks4 Science
 14 Key Calculations Combined Science Ppt Download
14 Key Calculations Combined Science Ppt Download
 Atomic Mass Vce Chemistry
Atomic Mass Vce Chemistry

 Relative Formula Mass
Relative Formula Mass
![]() Similar Images Stock Photos Vectors Of Relative Atomic Mass
Similar Images Stock Photos Vectors Of Relative Atomic Mass
 Topic 1 3 Chemical Reactions And Related Calculations
Topic 1 3 Chemical Reactions And Related Calculations
 Relative Molecular Mass Relative Formula Mass Solutions
Relative Molecular Mass Relative Formula Mass Solutions
 Obj C3
Obj C3



Belum ada Komentar untuk "How To Work Out Relative Formula Mass"
Posting Komentar